Ethical considerations and informed consent
This study adhered to the principles of the Declaration of Helsinki and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. The ethical principles of autonomy, confidentiality, and anonymity were considered. The Ethics Review Board of the Institute of Endocrine Sciences, Shahid Beheshti Medical University approved this study with ethics number IR.SBMU.ENDOCRINE.REC.1401.069. The research team's medical staff thoroughly explained the study protocol to each participant individually. Written informed consent was obtained from all participants; if they refused, they were excluded from the study. Informed consent included data collection while maintaining confidentiality principles, performance of necessary clinical tests, and permission for publication.
Lipid and glucose research in Tehran
This prospective cohort study is based on the Tehran Lipid and Glucose (TLGS) Cohort, a longitudinal population-based cohort aimed at investigating non-communicable diseases in Tehran, Iran. The TLGS survey used a multistage cluster random sampling method and targeted 15,000 adult residents of the eastern region of Tehran, Iran. Participants were followed every three years and data was collected from 1999 to 2021. We have previously discussed the details of TLGS.19,20.
All participants invited to the TLGS unit will be referred to an experienced physician after providing written consent. These doctors conduct interviews to gather participants' past medical history and complete a comprehensive 110-question questionnaire. The questionnaire covers a wide range of topics, including family history of NCDs, smoking habits, reproductive history, and assessment of physical activity. A simple physical examination, such as a physical measurement, will also be performed. Dietary data for one in ten of the participating families will be collected by a trained dietitian.
Educational level is divided into three groups: primary education (up to 6 years), secondary education (6 to 12 years), and tertiary education (12 years and above). Physical activity level was quantified using METS derived from an activity questionnaire, with less than 600 minutes per week indicating low activity. A trained physician taking anthropometric measurements will record her WC, weight, and height according to standard protocols and her BMI will be calculated accordingly. Participants will be asked to remain seated for 15 minutes, after which their blood pressure will be measured twice by a qualified physician using a standard mercury sphygmomanometer calibrated by the Iranian Institute of Standard and Industrial Research.
biochemical analysis
At the time of admission, individual characteristics are documented and a unique computer code is assigned. A 10 mL venous blood sample will be collected from all study participants between 7:00 AM and 9:00 AM after a 12-14 hour overnight fast. Blood samples are taken in a sitting position according to standard protocols and kept under standard laboratory conditions for one and a half hours.
All experimental kits are provided by Pars Azmon Inc., Iran. Serum total cholesterol and triglycerides (TG) are measured using enzymatic colorimetric tests using cholesterol esterase and cholesterol oxidase, and glycerol phosphate oxidase, respectively. HDL is measured after precipitating apolipoprotein B, which contains lipoproteins, using phosphotungstic acid. Assay performance is monitored every 20 tests using Precinorm, a lipid control serum. [normal range] and precipus [pathologic range] (Boehringer Mannheim, Germany; Cat. No. 1446070 for Precinorm, Cat. No. 171778 for Precipath).
Serum glucose concentration is assayed using an enzymatic colorimetric method with glucose oxidase technology, and fasting blood glucose (FBS) is assessed. Assay performance is monitored every 20 tests using Precinorm, a glucose control serum. [normal range] and precipus [pathologic range] (Boehringer Mannheim, Germany; Cat. No. 1446070 for Precinorm, Cat. No. 171778 for Precipath). A glucose standard (Cfas, Roche, Germany, catalog number 759350) is used to calibrate the Selectra 2 autoanalyzer on every day of laboratory analysis. All samples will be analyzed if internal quality controls meet acceptance criteria. The inter-assay and intra-assay coefficients of variation are both 2.2% for serum glucose and 0.6% for TG.19. Serum creatinine (Cr) is measured according to the standard colorimetric Jaffe-Kinetic reaction method (Pars Azmon Inc, Tehran, Iran).
Study design and exclusion criteria
This study included the available demographic data (age, gender), clinical tests (FBS, HDL, TG, Cr), smoking status, education, physical activity, and hemodynamic indicators (systolic and diastolic blood pressure). Data were collected from accessible regions. TLGS database. Participants completed a sixth follow-up every 3 years, resulting in at least 18 years of prospective observation.
We selected participants for the study from the TLGS cohort participant pool based on the following criteria: availability of valid data at the TLGS cohort stage and presence of glomerular filtration rate covariates or target variables. Exclusion of participants with missing values (GFR) were younger than 20 years, diagnosed with cancer or receiving corticosteroid treatment during the study period (Figure 1). From this enrollment cohort, we excluded participants who met any of the following criteria: (1) eGFR < 60 mL/min at baseline; (2) Follow-up period less than 1 year. (3) lack of conclusive data on metabolism and renal function;
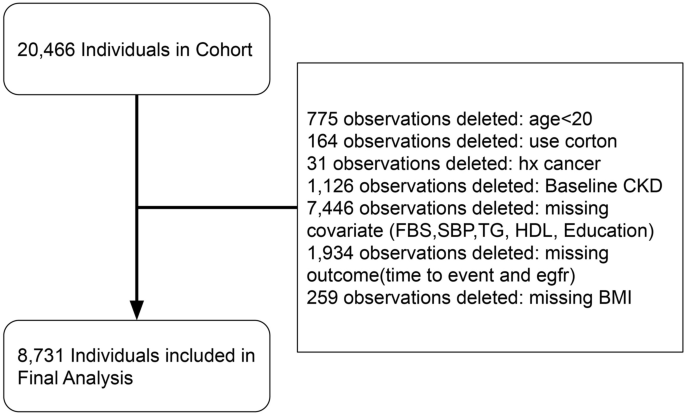
Patient flow diagram for patients and individuals included in the final analysis.
Purpose and results
This study aimed to answer the following questions:
-
Main objective 1: Evaluate the risk of developing CKD among four obese metabolic phenotypes.
-
Main objective 2: Determine the impact of participant state transitions on CKD risk.
-
Extension Study 1: Evaluate the adjusted impact of each component of metabolic health on CKD risk.
-
Extension study 2: In the context of defining obesity and metabolic health, what is the impact of a number of metabolic health factors on CKD risk in the presence and absence of obesity?
-
Extension Study 3: Regarding the definition of obesity and metabolic health, what is the agreement between using WC and BMI to define obesity?
The primary outcome of the study was the occurrence of CKD, defined as two GFR < 60 mL/min/1.73m2 in two periods of the TLGS cohort, separated by 3 years. The CKD-EPI formula was used to calculate GFR. This is:twenty one:
$$GFR=141 \times {{\text{min}}(\frac{Scr}{k}, 1)}^{\alpha } \times {{\text{max}}(\frac{Scr}{ k}, 1)}^{-1.209}\times {0.993}^{age}\times 1.018[if \;female]$$
where Scr is serum creatinine, κ is 0.7 for women and 0.9 for men, α is -0.329 for women and -0.411 for men, min is the minimum value, and max is the maximum value.
Definition of four phenotypes of metabolic health, obesity, and their interactions
The definition of metabolic health varies across studies and is controversial15. This study utilized a previously validated definition of metabolic health based on an evidence-based approach combined with expert opinion using the Delphi method.15. The consensus among experts to exclude waist circumference (WC) from the definition of metabolic health is consistent with the National Cholesterol Education Program Adult Treatment Panel III.twenty two. Therefore, metabolically unhealthy status in this study was defined as the presence of three or four of the following criteria:
-
(1)
Decreased HDL (<40 mg/dL in men and <50 mg/dL in women).
-
(2)
High TG (≥150mg/dL).
-
(3)
High FBS (>100 mg/dL) or use of antiglycemic oral medications for glycemic control.
-
(Four)
Have a systolic blood pressure of 130 mmHg or higher, or a diastolic blood pressure of 85 mmHg or higher, or are taking antihypertensive medications.
Obesity was defined as BMI >30 kg/m32. For the purpose of investigating the concordance between obesity and metabolic health and toileting and metabolic health, abnormal toileting was defined as 95 cm or more according to gender-specific cut-off points for Iranian adults.twenty three. Based on obesity status and the presence of components of the metabolic syndrome, participants were divided into four main groups:
-
MH-NO phenotype: This refers to individuals who exhibit a metabolically healthy profile and are free of obesity (metabolically healthy, non-obese: MH-NO).
-
MU-NO Phenotype: This category includes individuals with a metabolically unhealthy profile but who are not obese (Metabolically Unhealthy, Not Obese: MU-NO).
-
MH-O phenotype: This group consists of individuals with a metabolically healthy profile but who are obese (metabolically healthy, obese: MH-O).
-
MU-O Phenotype: This phenotype pertains to individuals who have a metabolically unhealthy profile and also have obesity (Metabolically Unhealthy, Obese: MU-O).
statistical analysis
Statistical analyzes were performed using Stata (StataCorp. 2015. Stata statistical software: Release 14, College Station, TX: StataCorp LP.). A p value of 0.05 or less is considered statistically significant. Baseline characteristics of participants were expressed as mean, standard deviation, median, interquartile range (IQR) for continuous variables, and frequency (%) for ordered variables. Participants' baseline characteristics between the four groups were compared by Student's t test and chi-square test for continuous and ordinal variables, respectively. We used the Cox proportional hazards test to analyze the association between the obesity phenotype and the metabolically unhealthy profile associated with CKD. Survival time was defined as the interval between study entry and appearance of CKD or censoring. Additionally, event time was defined as the survival half-time between the first diagnosis of CKD and the last normal test result. Univariate Cox regression analysis was performed for all confounders such as age, gender, BMI, and smoking.
For multivariate regression model analysis, additional variables with p-values less than 0.2 in univariate studies were considered. The first statistical model (unadjusted) showed the crude oil rate. The second model (adjusted for age and gender) adjusted for age and gender. The third model (fully adjusted) adjusted for age and gender (female, male), and the third analysis adjusted for age, gender, smoking (smokers, nonsmokers), and education (primary education, undergraduate , graduate students).and physical activity (task metabolic equivalent < 600、> 600). The Schoenfeld residual test was used to examine the proportional hazards assumption of the Cox model. Cumulative incidence of CKD was calculated as new cases during follow-up divided by individual time at risk. Additionally, the impact of each component on the overall cohort was assessed after adjusting for covariates and other constructs.
Finally, the three hazard ratios and their corresponding 95% confidence intervals (expressed as HR) [lower confidence interval, upper confidence interval] CKD incidence rates were calculated using three Cox tests: (1) unadjusted, (2) age- and sex-adjusted, and (3) fully adjusted (referred to as adj-HR) (age, sex, smoking, education, physical activity). For reports of HR stratified by gender, the stratification variable was excluded from the adjusted model.