Information source
In this retrospective cohort study, routine electronic medical records were obtained from the Hong Kong Hospital Authority (HKHA). The Hospitals Authority is the statutory body that manages all public hospitals and their outpatient clinics in Hong Kong. This service is available to all Hong Kong residents (more than 7.2 million people) and covers approximately 80% of routine hospitalizations.38. The electronic medical records in the HKHA database consist of disease diagnoses recorded during planned and unscheduled doctor's visits in inpatient and outpatient hospitals and emergency visits, and include all users of public health services in Hong Kong. Now you can get all your medical records in a timely manner. To determine mortality in this study, records were obtained from the Hong Kong Death Registry. Information on vaccination status was provided by the Hong Kong Special Administrative Region Government Department of Health, and records of confirmed cases of SARS-CoV-2 infection were obtained from the Hong Kong Special Administrative Region Government Health Protection Center. And Hong Kong. An anonymized unique patient ID was used to integrate these databases. These population-based databases have been used in previous studies on long-term sequelae of COVID-19 infection, safety monitoring and efficacy of COVID-19 vaccines.3,6,38,39,40,41,42.
Study design and population
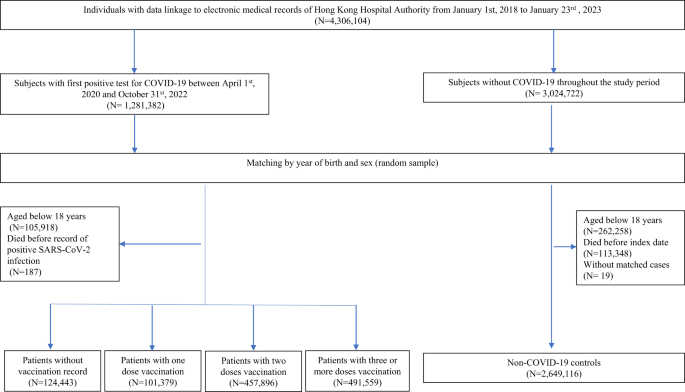
Individuals who linked their data to the Hong Kong Hospital Authority's electronic medical records between January 1, 2018 and January 23, 2023 were eligible for this study. A cohort study was conducted to assess the risk of health effects among SARS-CoV-2 infected and uninfected patients aged 18 years and older. Patients with SARS-CoV-2 infection (confirmed by rapid antigen test) [RAT] or polymerase chain reaction [PCR] Positive SARS-CoV-2 tests with accurate births throughout the study period Matched to unrecorded uninfected controls. Year and gender. All individuals with the same birth year and sex without a positive test record were selected as matched controls. Patients infected with SARS-CoV-2 are (1) unvaccinated (0 doses), (2) incompletely vaccinated (1 dose), (3) fully vaccinated (2 doses), (4) additionally vaccinated. vaccination (≥3 doses depending on number of BioNtech or CoronaVac vaccines received before first SARS-CoV-2 infection). The index date for SARS-CoV-2 infected patients was defined as the date of first diagnosis of SARS-CoV-2 infection. The same index date was assigned as a pseudo-index date to randomly selected matched matched controls.
All subjects were followed from the index date until the date of death, date of outcome, SARS-CoV-2 reinfection, or end of separate observation periods of 30, 90, 180, 270, and 365 days post-infection. I did. The earlier of the index date or the end of the study period, January 31, 2023.
Anonymized longitudinal clinical medical data from 2016 onwards and the first date of data availability were obtained from the HKHA for all subjects. Relevant data included baseline demographics (gender, age, Charlson Comorbidity Index). Pre-existing diseases captured by clinical diagnosis codes (cardiovascular disease, cerebrovascular disease, respiratory disease, chronic kidney disease, liver disease, rheumatoid arthritis and malignant tumors; Supplementary Table 1), long-term medication history (renin-angiotensin drugs) , beta-blockers, calcium channel blockers, diuretics, nitrates, lipid-lowering drugs, insulin, antidiabetic drugs, oral anticoagulants, antiplatelet drugs, immunosuppressants) and COVID-19 vaccination before the index date. situation.
This study was reported in accordance with the Reporting of Studies Conducted Using Routinely Collected Observational Data (RECORD), an extension of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Results of clinical diagnosis
The results of this study demonstrate the clinical sequelae associated with SARS-CoV-2 infection, including the incidence of major cardiovascular disease (a composite of stroke, heart failure, and coronary heart disease), stroke, and myocardial disease. selected based on previous evidence regarding the risk ofInfarction (MI), heart failure, atrial fibrillation, coronary artery disease, deep vein thrombosis (DVT), chronic lung disease, acute respiratory distress syndrome, stroke, end-stage renal disease, acute kidney injury, pancreatitis, cardiovascular disease, and all causes. death1, 8, 9, 10, 43. Outcomes were identified based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM, Supplementary Table 1).
statistical analysis
Inverse probability processing weighting (IPTW)44 Age, gender, Charlson Comorbidity Index (CCI), history of use of other classes of drugs (renin-angiotensin drugs, beta-blockers, calcium channel blockers, diuretics, nitrates, lipid-lowering drugs, insulin, antidiabetic drugs) , oral anticoagulants), antiplatelet agents, immunosuppressants), and the number of hospitalizations and physician visits within 1 year of the index date were applied to account for potential confounders. We estimated the standardized mean difference (SMD) between the case and control groups, and considered SMD ≤ 0.1 as a sufficient balance between the case and control groups.45. Subjects with a history of the outcome of interest were excluded from analyzes for the specific condition while still being considered at risk for other disease outcomes. Incidence rates (per 1000 person-years), hazard ratios (HRs), and 95% confidence intervals (CIs) for each outcome were calculated for each observation period using Cox proportional hazards regression for COVID-19 and non-COVID-19 cohorts. were estimated separately among the infectious disease cohorts. . Sensitivity analyzes were performed including only individuals with positive PCR SARS-CoV-2 screening test results and cases of SARS-CoV-2 infection by Omicron waves in Hong Kong.46, patients who were not vaccinated against COVID-19 and a control group with the same vaccination status adjusted for variants of SARS-CoV-2 that are more likely to cause infection. However, patients who received their last SARS-CoV vaccination more than 6 months ago are excluded. 2 Infectious diseases due to decreased immunity after vaccination47,48the false discovery rate was controlled to 0.05 using the Benjamin-Hochberg procedure.49. Lung cancer, brain cancer, and lymphoma, which are thought to have a long latency period, were included as negative control results to detect possible testing bias.Subgroup analyzes were predefined considering risk factors for post-COVID-19 conditions50. Patients were stratified by (1) age (≤65, >65), (2) gender, and (3) Charlson Comorbidity Index (CCI; <4, ≥4).
All statistical analyzes were performed using R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria). All significance tests were two-tailed.a P Values less than 0.05 or 95% CI excluding 1.0 were considered to indicate statistical significance. At least two researchers (ICHL, RZ, EYFW) independently performed each statistical analysis for quality assurance.
data access
EYFW and ICKW had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
ethical approval
Ethical approval for this study was granted by the Institutional Review Board of the University of Hong Kong/HA Hong Kong West Cluster (UW20-556 and UW21-149) and the Hong Kong Ministry of Health (L/M21/2021 and L/M175/2022). Ta. Because patient confidentiality was maintained in this retrospective cohort study, informed consent from participants was waived.
Report overview
For more information on the study design, please see the Nature Portfolio Reporting Summary linked in this article.